报告人:新加坡国立大学 卢一新教授
邀请人:张兴国
报告题目:Amino Acid-Based Bifunctional Phosphines for Enantioselective Reactions
报告时间:2018年4月4日(星期三)上午10:00
报告地点:化材楼11B204
----------------------------------------------------------------------------------------------------------------------
卢一新教授简介
Prof. Yixin Lu studied chemistry and received his B.Sc. from Fudan University, and continued his graduate studies in Canada and obtained his Ph.D. in Organic Chemistry under the supervision of George Just from McGill University in 2000. He then carried out his postdoctoral research with Peter W. Schiller at Clinical Research Institute of Montreal, and subsequently worked as an RCMS fellow with Ryoji Noyori/Masato Kitamura at Nagoya University. In September 2003, He started his independent career and joined the National University of Singapore (NUS) where he is now a Full Professor. Currently, he is holding Vice-Deanship at Faculty of Science. He was the recipient of a number of awards, including: Singapore National Research Foundation (NRF) Investigatorship Award (2018); Asian Core Program (ACP) Lectureship awards to China, Japan, Korea and Taiwan (2009 to 2016); Young/Outstanding Scientist Award from Faculty of Science, NUS (2009, 2013); GSK-SNIC Award in Organic Chemistry (2013); Dean’s Chair Professorship (2013). He is an Editorial Advisory Board Member for Accounts of Chemical Research, and Editorial Board Member for Asian Journal of Organic Chemistry.
卢一新,新加坡国立大学教授,博士生导师,现任新加坡国立大学理学院副院长。毕业于复旦大学获理学学士学位,2000年获加拿大麦吉尔大学博士学位。早年跟随著名诺贝尔化学得主野依良治(Ryoji Noyori)从事博士后研究工作。2003年加入新加坡国立大学化学系,进行不对称催化及合成,以及药物化学的研究。十多年来,取得了许多开创性的研究成果。其中,率先在国际上进行氨基酸衍生的双官能团、多官能团催化剂,尤其是有机膦催化剂的研究,及其拓展其在不对称合成中的应用。多年来在国际顶级期刊发表论文一百四十余篇,其论文被国际同行大量援引,很多工作被德国SYNFACTS杂志作为亮点文章进行报道,具有极高的国际知名度。由于卢教授丰硕的研究成果,他荣获了包括“Singapore National Research Foundation Investigatorship Award”“新加坡国立大学院长讲席教授”,和“新加坡国家化学会-葛兰素史克有机化学奖”在内的诸多奖项。此外, 卢教授注重工业化应用,其首次报道的氨基酸二肽叔膦催化剂已被日本东京化成商业化,他也曾获得著名跨国制药公司“葛兰素史克”工业研究奖项。卢教授现任Accounts of Chemical Research国际顾问编委委员。
Selected Recent Publications:
Ni, H.; Tang, X.; Zheng, W.; Yao, W.; Ullah, N.; Lu, Y. “Enantioselective Phosphine-Catalyzed Formal [4+4] Annulation of a,b-Unsaturated Imines and Allene Ketones: Construction of Eight-membered Rings”, Angew. Chem. Int. Ed. 2017, 56, 14222.
Yao, W.; Dou, X.; Wen, S.; Wu, J.; Vittal, J. J.; Lu, Y. “Enantioselective Desymmetrization of Cyclohexadienones via an Intramolecular Rauhut–Currier Reaction of Allenoates”, Nat. Commun. 2016, 7, 13024.
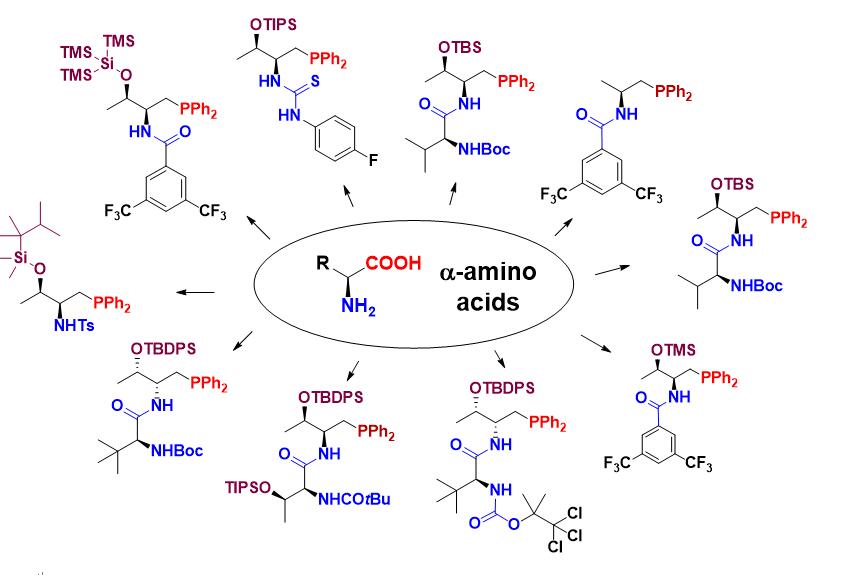
Wang, T.; Han, X.; Zhong, F.; Yao, W.; Lu, Y. “Amino Acid-derived Bifunctional Phosphines for Enantioselective Transformations”, Acc. Chem. Res. 2016, 49, 1369.
Dou, X.; Lu, Y.; Hayashi, T. “Base Free Conditions for Rhodium-Catalyzed Asymmetric Arylation to Produce Stereochemically Labile α-Aryl Ketones”, Angew. Chem. Int. Ed. 2016, 55, 6739.
Han, X.; Chan, W.-L.; Yao, W., Wang, Y.; Lu, Y. “Phosphine-mediated Highly Enantioselective Spirocyclization with Ketimines as Substrates”, Angew. Chem. Int. Ed. 2016, 55, 6492.
Wang, T.; Yu, Z.; Hoon, D. L.; Phee, C. Y.; Lan, Y.; Lu, Y. “Regiodivergent Enantioselective g-Additions of Oxazolones to 2,3-Butadienoates Catalyzed by Phosphines: Synthesis of a,a-Disubstituted a-Amino Acids and N,O-Acetal Derivatives”, J. Am. Chem. Soc. 2016, 138, 265.
Yao, W.; Dou, X.; Lu, Y. “Highly Enantioselective Synthesis of 3,4-Dihydropyrans through a Phosphine-Catalyzed [4+2] Annulation of Allenones and b,g-Unsaturated a-Keto Esters”, J. Am. Chem. Soc. 2015, 137, 54 (highlighted in SYNFACTS 2015, 201).
Han, X.; Yao, W.; Wang, T.; Tan, Y. R.; Yan, Z.; Kwistkowski, J.; Lu, Y. “Asymmetric Synthesis of Spiropyrazolones via Phosphine-Catalyzed [4+1] Annulations”, Angew. Chem. Int. Ed. 2014, 53, 5643.
Jiang, C.; Lu, Y.; Hayashi, T. “High Performance of a Palladium-Phosphinooxazoline Catalyst in Asymmetric Arylation of Cyclic N-Sulfonyl Ketimines”, Angew. Chem. Int. Ed. 2014, 53, 9936.
Wang, T.; Yao, W.; Zhong, F.; Pang, G. H.; Lu, Y. “Phosphine Catalyzed Enantioselective g-Addition of 3-Substituted Oxindoles to 2,3-Butadienoates and 2-Butynoates: Use of Prochiral Nucleophiles”, Angew. Chem. Int. Ed. 2014, 53, 2964.
Amino Acid-Based Bifunctional Phosphines for
Enantioselective Reactions
This talk is focused on our recent efforts on the development of bifunctional phosphines based amino acid structural motifs. Our phosphine catalysts can be readily prepared, and they are also very stable and structurally highly tunable. We have successfully applied these phosphine catalysts to asymmetric (aza)-Morita-Baylis-Hillman (MBH) reactions, various [3+2] cycloadditions, [4+2] cycloadditions, [4+1] annulation, [4+4] annulation, allylic alkylations, Michael addition, and g-addition reactions. The details of the above studies will be presented.